Nanoparticles are receiving plenty of attention nowadays in both the societal and scientific spheres. Some of these particles have existed in the natural state forever yet currently constitute a real source of technological breakthroughs across a wide array of fields: health, food, information science, transportation, to name a few. From the definition of a nanomaterial through the challenges inherent in its characterization, discover the key underlying technological and societal concerns in this special report prepared by our experts.
While some 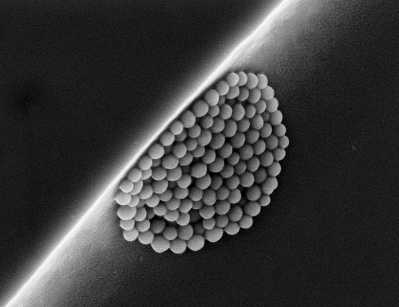 nanoparticles have existed in the natural state forever, they now represent a viable source of technological breakthroughs whose use affects a broad cross-section of fields: from energy to health, and finding their way into food, the information sciences and transportation. According to an inventory drawn up within the scope of the initiative called Project on Emerging Nanotechnologies, nanomaterials were already present in more than 1,300 commercial products in 2011.
nanoparticles have existed in the natural state forever, they now represent a viable source of technological breakthroughs whose use affects a broad cross-section of fields: from energy to health, and finding their way into food, the information sciences and transportation. According to an inventory drawn up within the scope of the initiative called Project on Emerging Nanotechnologies, nanomaterials were already present in more than 1,300 commercial products in 2011.
By offering industrialists new properties (optical, electrical, magnetic, etc.) that only appear at the nanometric scale, nanoparticles have become ubiquitous in everyday products. For example, they help improve product performance (more lightweight and stronger at the same time), contribute properties (antibacterial, UV filter, texture) and actually reduce the quantities needed of other additives.
Multiple definitions, a source of confusion
At present, an international consensus has been reached regarding the definition of a nanoparticle: "a material whose three characteristic external dimensions all lie at the nanoscale (i.e. between 1 and 100 nm, ISO)". On the other hand, the notion of nanomaterial proposed within the regulatory domain is still open to debate. Various nanomaterial definitions coexist today in Europe, each with a number of specificities depending on the industrial sector. The recommended definition 2011/696/EU (adopted in European regulations on biocides and medical devices, as well as for the mandatory French declaration R-Nano) includes a threshold in terms of proportion of number of nanoparticles present in the substance. For their part, cosmetic and food regulations have not included any such proportion. Standardization by the European Commission in order to establish the meaning of nanomaterial is expected during 2018 for purposes of clarifying the situation, some four years beyond the time initially scheduled.
On this very topic, the European Commission has opened a public consultation, with input accepted through October 13, 2017.
Demonstrating the nano nature of a substance remains first and foremost a measurement challenge
 Determining the nano nature of a substance, as intended by the recommended European definition, requires measuring the proportion of primary particles of a size less than 100 nm relative to all particles present in the substance, regardless of whether they are isolated or combined into mounds (clusters/aggregates). The act of measuring primary particles within the mounds recognizes that during their life cycle, these mounds are capable of leaching out isolated nanoparticles.
Determining the nano nature of a substance, as intended by the recommended European definition, requires measuring the proportion of primary particles of a size less than 100 nm relative to all particles present in the substance, regardless of whether they are isolated or combined into mounds (clusters/aggregates). The act of measuring primary particles within the mounds recognizes that during their life cycle, these mounds are capable of leaching out isolated nanoparticles.
Many analytical techniques yield this information, yet no instrument can claim to be perfect. Significantly different results can thus be obtained depending on the technique employed, each one of which is associated with its own set of limitations, notably due to: sensitivity, confusion between a mound of nanoparticles and a primary particle larger than 100 nm, and the decision over whether or not to include the impact of particle shape (spherical, elongated in the form of small sticks or even plates). It thus appears crucial to rely upon techniques adapted to the specific characteristics of the substance being analyzed. The European project NanoDefine will be proposing, as of end of October 2017, the NanoDefiner E-Tool, a freeware interface that identifies the most pertinent technique(s) suited to the substance under characterization, as well as those techniques to be avoided.
The approach recommended nowadays to enhance information reliability consists of cross-referencing the results derived using several of the analytical techniques available, in noting however that scanning electron microscopy (SEM) remains the benchmark within the international scientific community in the case of doubt. Considerable expertise in preparing samples and interpreting the images obtained is nonetheless a necessary precondition to extracting high-quality results.
High-quality measurements as an essential prerequisite
Whether in search of optimizing the performance of products containing nanotechnologies or evaluating the risks potentially associated with such use, industrial actors and government agencies alike are required to base their decisions on measurement data whose quality and comparability are a given.
For industry, t he development of nanotechnologies takes place within the scope of a responsible innovation plan, in which risk evaluation has been anticipated as of the design phase. This sequence must take into account the key stages in the life cycle of a product from its manufacturing, then use and extending through to its waste disposal. From one target application to the next, two levers always serve to reduce the potential risk associated with the implementation of nanomaterials:
he development of nanotechnologies takes place within the scope of a responsible innovation plan, in which risk evaluation has been anticipated as of the design phase. This sequence must take into account the key stages in the life cycle of a product from its manufacturing, then use and extending through to its waste disposal. From one target application to the next, two levers always serve to reduce the potential risk associated with the implementation of nanomaterials:
- exposure mitigation: by characterizing the nanoparticles released in various situations (workstation, product wear, migration, emissions during a fire or waste processing by means of incineration, etc.);
- toxicity reduction: by modifying certain physicochemical properties of the nanomaterials.
In both cases, it is necessary to gain access to reliable and comprehensive measurement data. However, the specific properties of nanomaterials depend on a wide array of parameters (size, particle size distribution, shape, specific surface area, charge, surface chemistry, etc.), all of which need to be well controlled. A reliable determination of these characteristics, as a prerequisite to maximizing the use of these materials, remains a very arduous task due to the multitude of analytical techniques available in the market, as well as to the lack of an ideal measurement method. Consequently, special attention must be paid to these issues in order to optimize product development-related costs and smooth the transition from the laboratory to a pilot production line. Inspecting raw materials and verifying potential batch effects also constitute a critical stage.
Grounded in reliable nanometric-scale benchmarks
Through current participation in just under ten R&D studies and projects dedicated to these topics, LNE is a leading actor in the field: the laboratory is developing reference measurement protocols and a full set of tools (instruments, standards, test benches) in the aim of generating measurement results traceable back to the International System of Units (SI) from a metrological perspective, which remains the lone condition capable of ensuring their comparability among different laboratories.
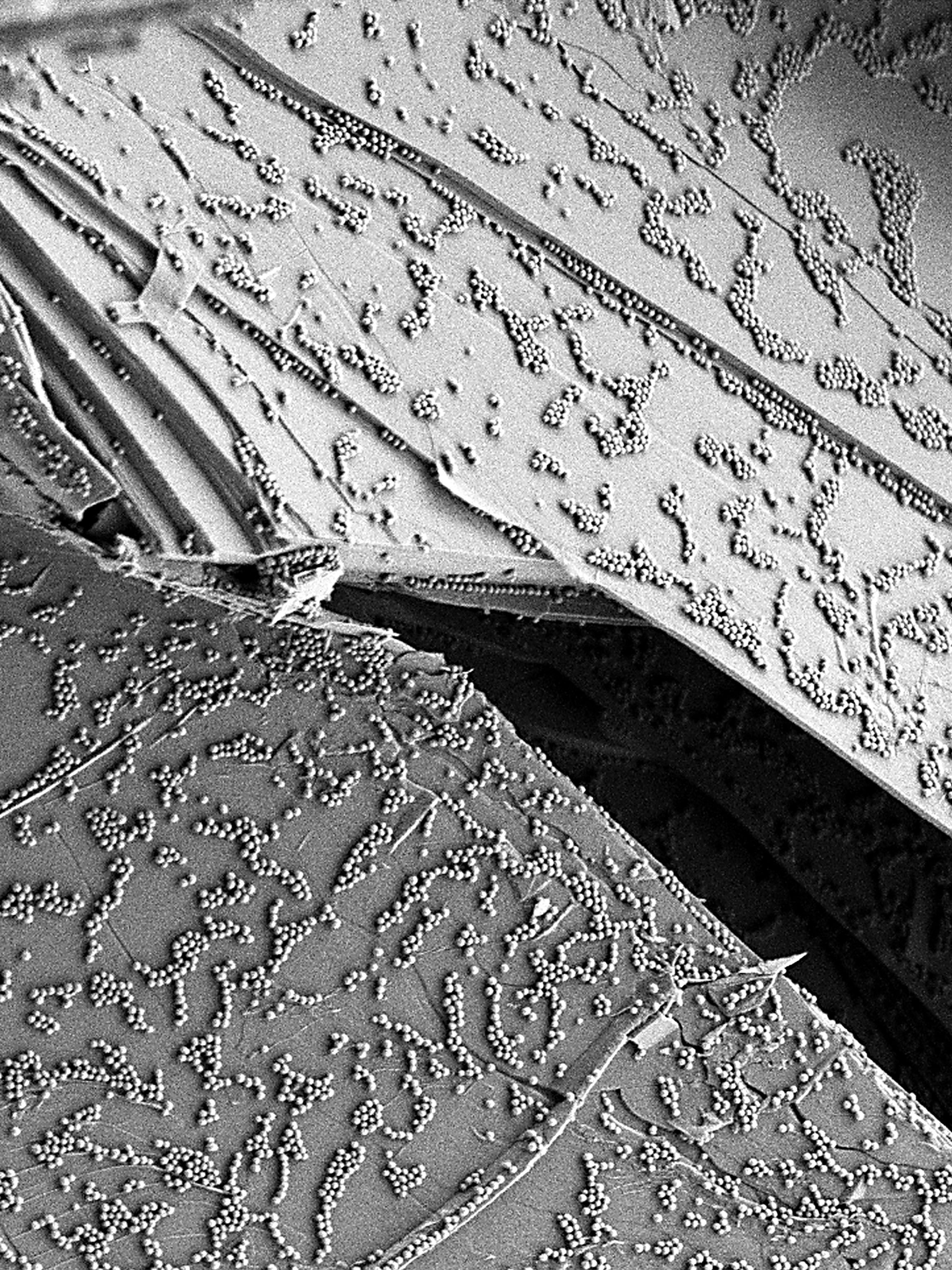 The methods under development are intended to provide high-quality data for an extensive spectrum of nanomaterials (nanoparticles, nanotubes, graphene, etc.) and media, from environmental matrices (air, water, soot) to biological matrices (culture media, cells, etc.) while not overlooking industrial matrices (food products, cosmetics, paints, etc.). Along these lines, LNE has been tasked with coordinating the NANOMET project financed by the Energy Directorate, with the goal of assisting French small and medium enterprises (SME) working in these areas through both furnishing reference protocols to characterize their products (downloadable on the project site) and contributing to the NanoDefine project, a showcase initiative sponsored by the European Commission in the field of nanomaterial characterization, as per the recommended definition. LNE is also responsible for coordinating activities of the nanoMetrology Club, a network comprising over 390 actors, one-third from industry (including some 50 SME focused on nanomaterial characterization problems). This network has provided the backdrop for conducting interlaboratory comparisons intended to assess the performance of various measurement techniques.
The methods under development are intended to provide high-quality data for an extensive spectrum of nanomaterials (nanoparticles, nanotubes, graphene, etc.) and media, from environmental matrices (air, water, soot) to biological matrices (culture media, cells, etc.) while not overlooking industrial matrices (food products, cosmetics, paints, etc.). Along these lines, LNE has been tasked with coordinating the NANOMET project financed by the Energy Directorate, with the goal of assisting French small and medium enterprises (SME) working in these areas through both furnishing reference protocols to characterize their products (downloadable on the project site) and contributing to the NanoDefine project, a showcase initiative sponsored by the European Commission in the field of nanomaterial characterization, as per the recommended definition. LNE is also responsible for coordinating activities of the nanoMetrology Club, a network comprising over 390 actors, one-third from industry (including some 50 SME focused on nanomaterial characterization problems). This network has provided the backdrop for conducting interlaboratory comparisons intended to assess the performance of various measurement techniques.
Moreover, LNE's contribution at a symposium organized in May 2017 by the European Commission on the topic of nanomaterials in the food processing sector attests to the Laboratory's renowned expertise at the European level.
Cutting-edge equipment for characterization purposes
LNE's expertise and resources have placed it in a position to propose a full panoply of services, encompassing: technical and regulatory assistance and training, calibration operations dedicated to SEM and AFM microscopy techniques, characterization of the main critical properties of nanomaterials (whether correlated or not with regulatory requirements of the R-Nano or labeling type), evaluation of nanomaterial exposure at workstations, assessment of migration originating from packaging materials, and characterization of nanoparticle emissions during the thermal degradation of nanocharged composite materials.
The platforms called "CARMEN" (for Metrological Characterization of Nanomaterials) and "MONA" (On- and Off-line Metrology of Nanoaerosols) offer state-of-the-art tools at both the French and European levels. More specifically, they make it possible to identify and characterize the primary set of parameters and nanomaterial stability in a large number of matrices and products.
- The CARMEN platform combines various analytical resources for the purpose of characterizing:
- the size and size distribution of nanomaterials (Scanning Electron Microscope - SEM, Atomic Force Microscope - AFM, Dynamic Light Diffusion - DLS, pulse-counting Mass Spectrometry (spICP-MS), Flux-Force Fractionation Chain coupled with various detectors (A4F-UV-RI-MALS)
- their shape (SEM, AFM)
- their state of clustering/aggregation (SEM, DLS, BET)
- their specific surface area and porosity (BET instrument)
- their chemical composition and mass content in products (EDX, Mass spectrometry - ICP-MS)
- their crystalline structure (X-Ray diffraction - XRD)
- their surface charge (zeta potential).
- The MONA platform is dedicated to characterizing nano-objects in aerosol form. This platform is intended to ensure metrological traceability with respect to the International System of Units (SI) for these types of measurements, with the objective of guaranteeing the comparability of results. Reference protocols for generating aerosols with controlled characteristics and characterizing the size, size distribution and nanoparticle concentration in number or mass in air are developed at this facility. MONA features instruments that allow, among other things:
- generating reference aerosols from colloidal suspensions or powders (electrospray, atomizer, spark generator);
- characterizing the pulverunce of nanostructured powders (vortex-shaker);
- characterizing aerosols in real time or after extraction onto various supports (filters, microscopy grids, etc.), for a size range extending from a few nanometers to several micrometers. This step entails use of instruments dedicated to this type of real-time measurement, such as CPC particle counters, scanning electrical mobility spectrometers (SMPS) (3 nm < pd < 1 µm), a fast particle analyzer DMS (5 nm < pd < 2.5 µm), as well as optical spectrometers APS (0.6 µm < pd < 20 µm). A number of extraction systems (e.g. NAS, MPS, Nano ID) enable analyses to be conducted using off-site techniques (SEM, ICP-MS, etc.).
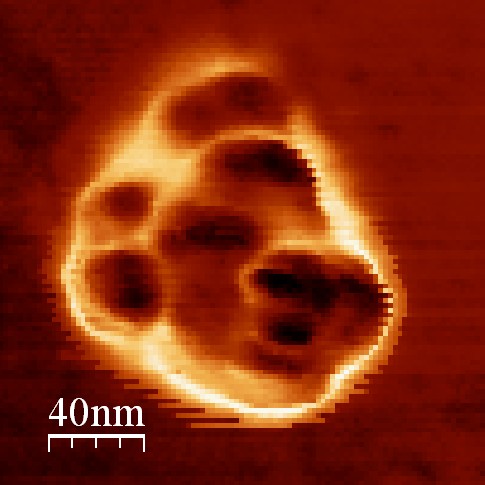
 The metrological AFM (atomic force microscope), a French reference instrument for dimensional measurements at the nanometric scale, offers French actors their first opportunity to deploy certified reference facilities, with some standards having been specially designed by the Laboratory for their specific microscopes, in pursuit of metrologically-traceable measurements.
The metrological AFM (atomic force microscope), a French reference instrument for dimensional measurements at the nanometric scale, offers French actors their first opportunity to deploy certified reference facilities, with some standards having been specially designed by the Laboratory for their specific microscopes, in pursuit of metrologically-traceable measurements.
Moreover, an instrumented test bench has been installed to simulate all types of thermal degradations affecting nanocharged products, for the purpose of quantifying the (nano)particles generated and then extracting them in a representative manner so as to facilitate characterization, thus setting the stage to develop products while controlling risks!

Nicolas FELTIN
Researcher - Expert in nanometrology
What are industry's obstacles to improving the performance of their nanocharged products?
The performance of a product containing nanomaterials (called nanocharged) is closely correlated with the product's functional properties. Such properties are highly dependent on the main physicochemical parameters characterizing a nano-object, e.g. size, shape, load. Measuring these various parameters remains, to this day, a real metrological challenge, in knowing the difficulties often faced by industry when deploying a reliable quality control system.
Such obstacles must hinder studies of their toxicity?
You're exactly right. Because here again, evaluating the toxicity of nanoparticles presumes that they have been perfectly characterized ahead of time. This is LNE's emphasis, in accordance with the guidelines issued in ISO/TR 13014, as a preliminary to any toxicity study.
What about additional considerations?
Yes indeed, namely exposure. A product may contain nanoparticles that, despite displaying a certain level of toxicity, will never be exposed to us. In this case, the risk would be zero. In various R&D projects, like EMANE (financed by the ADEME Agency) or INNOVIP (financed by Europe's Horizon 2020 program), LNE has helped conduct such an evaluation. This effort is followed up by a search for levers to limit the exposure to nanoparticles, at each step in a product's life cycle.
LNE webinars dedicated to nanomaterials






