As of 1 January 2021, the European CE marking regulations no longer apply in the UK. However, CE marked products can continue to be placed on the UK market until 31 December 2024, with the exception of medical devices for which the deadline is 1 July 2023 and construction products autorised until 30 june 2025.
What is the UKCA marking?
In Great Britain, the European regulation is replaced by a British regulation which provides for the UKCA marking. This marking applies to most products previously subject to CE marking. The technical requirements that will have to be met - and the conformity assessment procedures and standards that can be used - are currently largely the same as for CE marking. Where conformity assessment procedures require the involvement of an assessment body, the body must be established in the UK and approved by the UK authorities.
Why choose LNE group?
In order to meet the challenges of our customers (maintain your activities on the UK market, be in regulatory compliance), the LNE group has created its subsidiary LNE-GMED UK, based in the UK, which operates in the following fields
- Measuring instrument and NAWI
- Noise emission by outdoor equipment
- Construction products
- Medical devices (Watch this space)
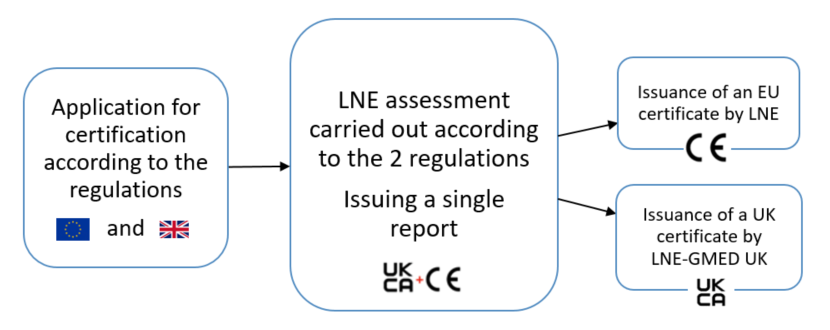
A joint evaluation process
The LNE Group is able to offer its customers and prospects the possibility of obtaining the certifications required to apply the UKCA mark. Given the similarities between European and British regulations, these certifications can be obtained through a joint assessment process.

The certification process and the rules inherent to the services of the LNE-GMED UK subsidiary to be followed are described in LNE-GMED UK Certification Rules
Contact
For any information request, please contact our customer service at: info@lne.fr
For enquiries about the validity of UKCA certificates issued, please contact us at: Ukenquiries@lne-gmed.com
For any additional impressions and suggestions, please contact LNE-GMED UK’s team at: UKcustomerfeedback@lne-gmed.com
All our teams are committed to taking customer feedback into consideration in order to continuously improve our service offering.





