Thanks to its specialist international subsidiaries, the Group helps its customers win new markets and control their subcontracting.
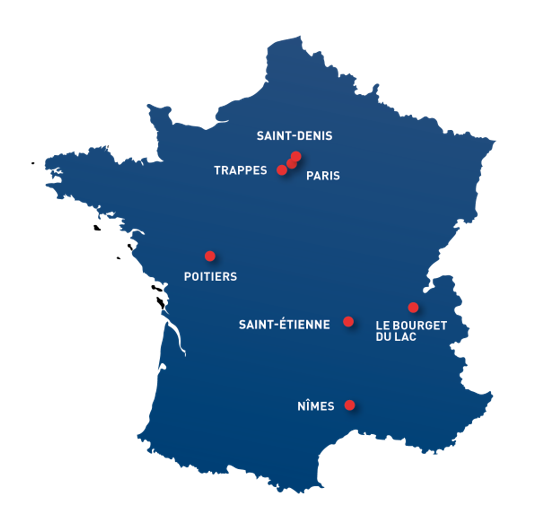
In France

- Paris
Groupe LNE - Head office, LNE Développement
Research, metrology, certification, training - Trappes
LNE - Research, expertise, metrology, testing - Nîmes
LNE - Expertise, metrology, testing, certification, training - Le Bourget-du-Lac
Certisolis - Testing and certification (photovoltaic panels) - Saint-Denis
LNE - Research, metrology - Poitiers
LNE – Metrology - Saint-Etienne
GMED - Certification, training (medical-health)
Subsidiaries in France and abroad
Groupe LNE has subsidiaries in France and abroad. These wholly-owned or jointly-owned subsidiaries enable Groupe LNE to deploy an agile strategy to support its customers as closely as possible to their needs and markets.
Certisolis
Certisolis is the only French laboratory dedicated to the testing and certification of photovoltaic panels. Innovation is dazzling in the photovoltaic sector; to meet these challenges Certisolis is investing and ramping up the skills of its teams by drawing on the resources of its parent companies. Certisolis is at the forefront of providing support for the photovoltaic revolution and the ecological transition.
sales growth by 2024
GMED
GMED SAS, a subsidiary of the LNE, is the benchmark certification body for medical devices. Its aim is to provide customers with a better service in a context of major changes in standards and regulations, while serving the health of our fellow citizens and the competitiveness of our industry. GMED's expertise is recognised internationally:
- since 2021, it has been one of the 6 European organisations to have been designated as a notified body under Regulation (EU) 2017/746 on in vitro diagnostic medical devices;
- it has been recognised as a partner notified body under the TCP III mutual recognition programme set up by the TFDA (Taiwan Food and Drug Administration); TCP III gives manufacturers access to the Taiwanese market
-
in 2024, GMED Asia was created: this new location is designed to better support manufacturers who produce their medical devices in Asia and wish to distribute them on markets covered by European regulations and the MDSAP.
Sales growth recorded by GMED SAS compared with 2023.
LNE-LP ASIA
Since the first half of 2021, Groupe LNE and Laboratoires Pourquery have pooled their expertise with the aim of providing even better support for manufacturers, importers and distributors of consumer goods manufactured in Asia. As a result, they now have a one-stop shop for a comprehensive range of tests covering 90% of the products on the market.





