In 1971, the mole – the unit of measurement for amounts of a substance – became the last of the seven units to be integrated into the International System of Units (SI). Defined in the past based on the number of atoms contained in 12 grams of carbon-12, the mole is among the four base units in the SI that were redefined on the basis of a physical constant at the 26th General Conference on Weights and Measures (CGPM) in November 2018.
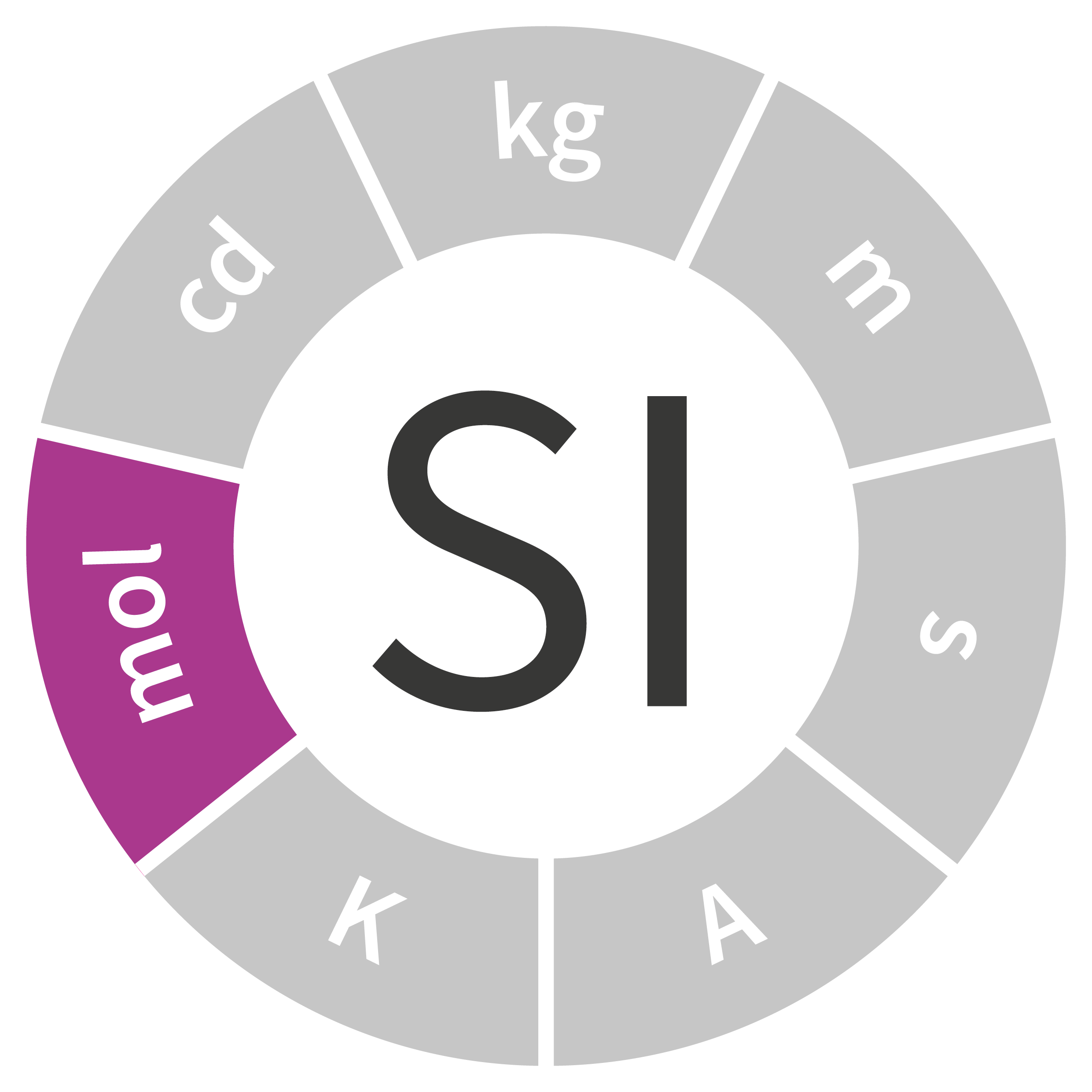
Official definition (1971, 14th CGPM)
Symbol: mol
Quantity: amount of substance
Units derived from the mole: mole per cubic meter, katal
Origins of the mole: Why a unit for chemistry?
The mole primarily derived from the need to define a unit that was specific to chemistry and resolve a number of issues related to the unit of chemical mass. With its introduction in 1971 as the seventh unit in the SI, the mole served as a recognized unit specifically for the field of chemistry. But it was only in 1993, with the founding of the Consultative Committee for Amount of Substance (CCQM) under the aegis of the International Bureau of Weights and Measures (BIPM), that the mole truly assumed an important role in connection with metrology, i.e., as a means of determining how the traceability of SI measurement results can be upheld. Since the early 1990s, chemical metrology has primarily evolved in tandem with growing demand in areas such as the environment, healthcare and food.
However, there has been an increasingly pressing need for more reliable chemical analyses in a wide variety of additional fields: manufacturing, which relies more and more heavily on measurements with established traceability; pharmaceuticals (for the dosage of active ingredients and their impurities); cosmetics (for the dosage of endocrine disruptors); manufacturers of measurement instruments (for calibration purposes with regard to new technology in particular, evaluation of sensor performance, etc.), and so on.

A major driving factor behind the growth of metrology in general and chemical metrology (a young branch of the discipline) in particular has been the adoption of standards and regulations designed to restrict the presence of harmful compounds in natural environments or food products, verify product conformity or enhance the quality of the results from biological examinations. When addressing the issue of human health and its cost to the community, it is important to have reliable results, in part so that prescriptions are not issued to healthy patients, but also to ensure that those stricken with a disease can be treated at the earliest possible stage.
A history of the mole from 1971 up to its redefinition in 2018
Chemical metrology plays an important role in analytical chemistry, with numerous and exceptionally wide-ranging applications for product analysis. It draws on the various branches of chemistry: organic, inorganic, gas, electrochemistry, biochemistry, etc. That extensive array of applications derives from the large number of elements and chemicals at play and the broad range of potential matrices: human serum, poisons, drinking water, aerosols, sunscreens and so on.
As we have seen, chemical metrology is one of the discipline’s more recent fields of study; indeed, the CCQM was only founded in 1993.
To address these growing needs, in 1971 the 14th CGPM adopted the mole as a base unit in the SI by defining it as follows:
“The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12.”
The definition goes on to state that “When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.”

The traceability of a mole does not involve physically preserving a primary standard; instead, it relies on a set of metrological tools, such as a mastery of the procedures, the use of calibrated instruments and validated procedures thanks to matrix reference materials, and estimates of measurement uncertainties. These tools make it possible to realize the national references.
In practice, measuring a number of moles often comes down to measuring a mass of the given element or chemical, or even a ratio of a mass or a quantity of substance.
“Mole vs. kilogram: why the need for a separate unit for chemistry?” (video in French only)
Up until the 26th CGPM in November 2018, the definition of a mole had not been changed since it was first added to the SI. The new definition is based on the Avogadro constant, a fixed numerical value, as follows:
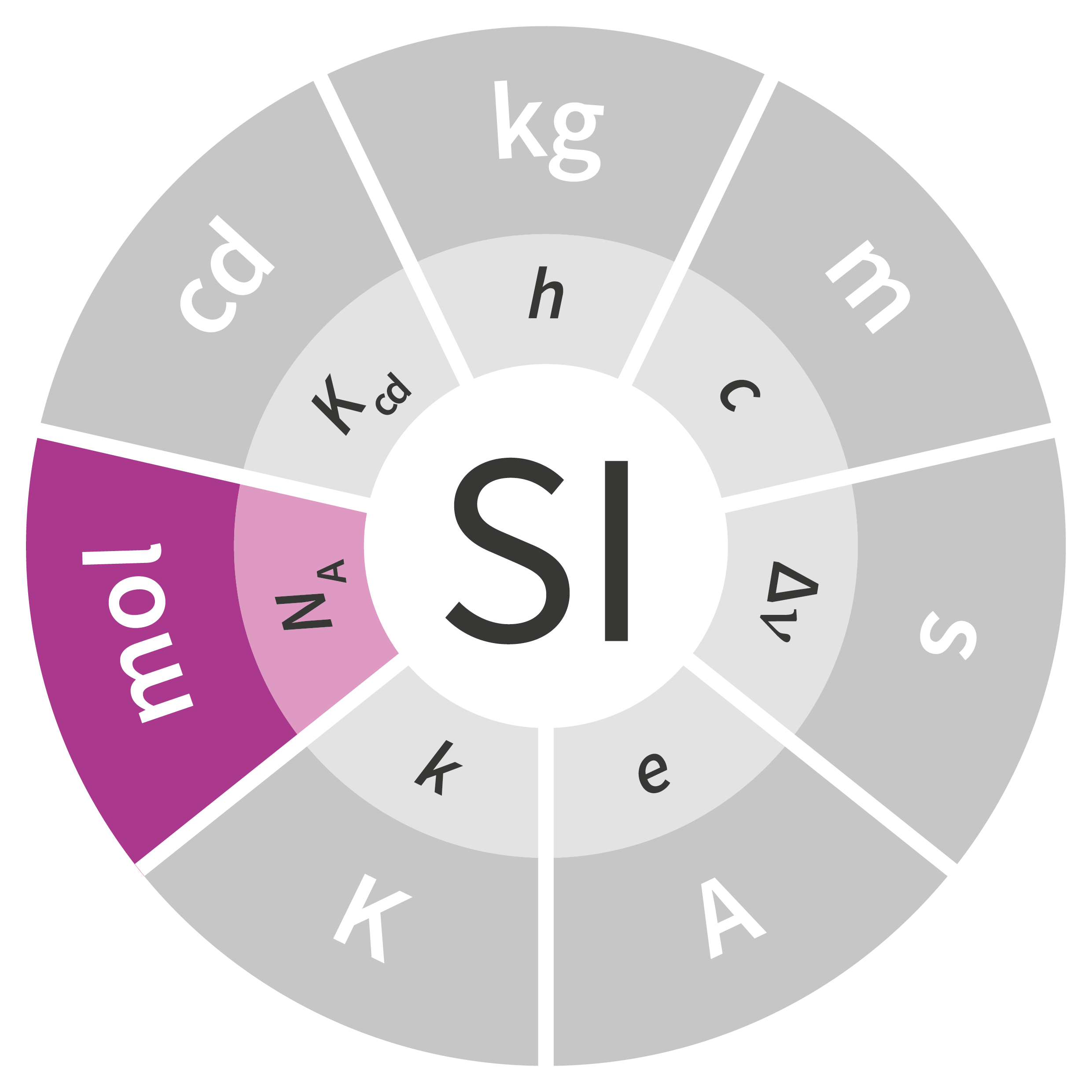 The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol–1 and is called the Avogadro number. The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol–1 and is called the Avogadro number. The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles.
“What are the benefits of redefining the mole?” (video in French only)
“The new definition of the mole: the Avogadro sphere experiment” (video in French only)
The mole – the unit for chemistry – is at the heart of health and climate change
Whether for monitoring changes in ocean acidity, measuring air pollution, determining the quantity of biomarkers in human serum or identifying drug residues in drinking water, analytical chemistry and chemical metrology in particular are at the heart of numerous environmental concerns and public health issues. Metrological resources, such as reference materials, device connections to the SI, estimates of measurement uncertainties and so on, provide access to reliable analyses. With regard to this, the LNE is developing gas reference materials that allow researchers to align the measuring equipment used to measure air quality, as well as reference materials for methane, nitrous oxide, carbon monoxide and carbon dioxide that are used to monitor greenhouse-gas emissions over time.
The acidification of our oceans receives less media attention than rising temperatures but nonetheless poses a significant ecological threat. Lower pH levels in seawater, caused by an accumulation of CO2, have disastrous consequences for marine biodiversity. To monitor that phenomenon, specialists must measure an array of parameters, including pH, with as little uncertainty as possible. In response, the LNE is testing the feasibility of a reference material for measuring seawater acidity, at the request of oceanographers seeking more reliable measurement results.





